THE CONSEQUENCES OF MITOCHONDRIAL DNA MUTATION ON NEURODEGENERATIVE DISEASES
Keywords:
Mitochondrial DNA, Neurodegeneration, Oxidative Phosphorylation, Reactive Oxygen Species, Mitophagy, Neuroinflammation.Abstract
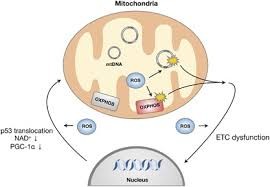
Mitochondrial DNA (mtDNA) mutations have been more and more embraced as key determinants in the pathogenesis of a range of neurodegenerative disorders, most notably Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), and Amyotrophic Lateral Sclerosis (ALS). Situated close to the electron transport chain, without the protection of histones, and with lower potential for repair, mtDNA is more susceptible to mutation than nuclear DNA. These mtDNA mutations, like large-scale deletions and point mutations, undermine oxidative phosphorylation by compromising the electron transport chain, reducing ATP generation and augmenting ROS production. The consequent energy deficit and oxidative stress severely deteriorate neuronal function and viability. In addition, mtDNA mutations negatively affect mitochondrial dynamics—deteriorating the delicate equilibrium of fission and fusion—and impair mitophagy, the critical process for the removal of defective mitochondria. These dysfunctions also sustain a vicious cycle of mitochondrial stress, leading to apoptotic cell death and the release of mitochondrial DAMPs (damage-associated molecular patterns) that trigger chronic neuroinflammation through pathways like NLRP3 inflammasome and cGAS-STING. This review integrates robust experimental and clinical evidence that connects mtDNA alterations with underlying neurodegenerative mechanisms and emphasizes the importance of addressing mitochondrial health in therapeutic strategies. Promising advances such as the use of mitochondrial antioxidants (e.g., MitoQ, CoQ10), new gene editing technologies (e.g., mitoTALENs, CRISPR), and mitophagy-inducing therapies offer a window of opportunity for the development of effective, disease-modifying therapies. With the prevalence of neurodegenerative diseases still increasing, an improved understanding of mtDNA-mediated mitochondrial dysfunction has the potential to revolutionize early diagnosis, prognosis, and therapeutic intervention in these disabling disorders.